Heart pathologies in cats are classified as diseases of older age. The most common cardiomyopathies are myocardial lesions characterized by an increase in the size of the heart muscle. Of several varieties, hypertrophic cardiomyopathy (HCM) is most often diagnosed - isolated myocardial damage with hypertrophy (thickening) of the ventricular walls. Dilated cardiomyopathy (DCM) is also common, in which there is a pathological enlargement (dilatation) of the heart chambers. The insidiousness of this disease is that it may not make itself known for a long time, suddenly attacking the pet with the full severity of the symptoms. Therefore, the owner needs to be especially careful not to miss the first signs of a developing illness in his pet.
Types of disease
Hypertrophic cardiomyopathy in cats is classified as primary or idiopathic. This means that it is an independent disease that is not a complication or symptom of another illness. It is presumably genetic in nature, so it can also be diagnosed in young individuals.
In addition, in more rare cases, HCM may also be secondary. In this case, it appears as a consequence of a pre-existing disease (diabetes, kidney dysfunction, thyroid disease).
The mechanism of pathology development
Hypertrophy of the walls of the ventricles significantly impairs the functioning of the heart, which leads to stagnation of blood in the atria. Weak blood flow causes circulatory hypoxia (oxygen starvation caused by poor circulation). Due to spasm of peripheral vessels, the risk of thrombosis increases. If the disease is not treated, it can lead to the development of heart failure, dangerous arrhythmias, and even cause the death of the animal.
Depending on the location, HCM is classified into the right or left ventricle. In cats, lesions of the left ventricle and interventricular septum are more common.
The pathological process slows down the growth of muscle tissue that forms the contractile system of the heart. The body compensates for this deficiency with connective tissue, which thickens the walls of the myocardium. Peculiar scars appear on the heart, reducing the size of the heart chambers. For the same reason, the elasticity of the organ decreases.
Etiology of hypertrophic cardiomyopathy in cats
Feline hypertrophic cardiomyopathy is divided into two types: primary (the origin is not fully understood) and secondary (as a consequence of some disease). Primary, in turn, can be obstructive and non-obstructive.
- Obstructive - high pressure is created in the cavity of the left ventricle due to an increase in the myocardium, blood flows into the aorta at a higher speed, this process resembles a whirlpool. Due to this vortex blood circulation, the leaflet of the mitral (bicuspid) valve spontaneously opens and closes.
- Non-obstructive - the same thing happens, only the high blood velocity does not affect the function of the bicuspid valve.
- Acquired (secondary) - directly related to age-related changes and concomitant diseases, which are characterized by changes in the myocardium. Causes may include disruption of the endocrine system, infectious diseases, toxic substances, and injuries. Such diseases rarely lead to severe manifestations of heart failure.
Primary cardiomyopathy can also be attributed to a genetic predisposition—the development of heart failure is hereditary. This disease is passed down from generation to generation to certain breeds of cats. Particular attention should be paid to such breeds as British, Scottish, Persian, Maine Coon, Sphynx and their mixed breeds. Occasionally, this disease occurs in outbred animals; here we mean genetic inheritance.
In the vast majority of cases, hypertrophic cardiomyopathy manifests itself at the very beginning of the animal’s life, starting from six months.
Cats diagnosed with cardiomyopathy are not allowed to continue breeding for offspring. This is the only prevention to reduce the risks of developing myocardial pathology that may occur in future generations.
Reasons for the development of the primary form
American researchers have found that the primary nature of the disease is due to a hereditary factor. They also concluded that Maine Coon and Ragdoll cats are the most predisposed to pathological mutations. Later, the following breeds were added to the risk group for the incidence of primary hypertrophic myocardiopathy: Persian, Sphynx and Abyssinian.
Currently, the fact that the presence of mutations in genes increases the likelihood of the disease is considered proven, although the number of sick cats with defective genes was only 40% of the total number that took part in the study. Therefore, it is possible that there are other hereditary disorders that increase the likelihood of the disease occurring.
The genetic nature of HCM is confirmed by cases of the disease in both parents and their children. It is based on inherited mutations in genes responsible for the production of the myosin protein, which is responsible for the contractile function of the myocardium.
The secondary (acquired) nature of the disease is caused by mutations of the same genes, which occur under the influence of various unfavorable environmental factors and as a result of improper care.
Causes of the secondary form of the disease
Cats of the following breeds are susceptible to secondary forms of the disease: British Shorthair, Siamese, Russian Blue and Siberian. Secondary cardiomyopathy can develop in them as a complication of a whole series of ailments.
1. Heart and lung diseases:
- congenital myocardial pathologies (bovine heart);
- infectious diseases of the heart muscle;
- arterial hypertension;
- pneumonia of various types.
2. Metabolic disorders:
- amyloidosis (a protein metabolism disorder characterized by pathological deposition of amyloid protein in tissues);
- hemochromatosis or pigmented cirrhosis (liver disease leading to impaired iron metabolism in the body).
3. Endocrinological diseases:
- acromegaly (abnormal production of growth hormone);
- hyperthyroidism (overactive thyroid gland).
4. Malignant neoplasms: lymphoma (oncological disease of the lymphatic system).
In addition, improper conditions for keeping the animal may be provoking factors:
- Consequences of long-term stress.
- Deficiency of essential microelements in the diet. This is especially true for the amino acid taurine. A sufficient amount of it has a beneficial effect on the heart muscle, reduces the load on the heart, and protects heart tissue from damage.
- Chronic intoxication, the source of which can be long-term drug therapy, exposure to household chemicals, and helminth infection.
Symptoms of the disease
Hypertrophic cardiomyopathy is often characterized by a long absence of symptoms. This course is explained by the good compensatory ability of the heart at a young age. But gradually the heart can no longer cope with the load, and severe symptoms develop in a short time. If treatment is not started in time, the animal will not live long.
Therefore, it is very important for the owner to take action as soon as he notices the first warning signs:
- lethargy, loss of interest in outdoor games, physical inactivity. Body temperature may be reduced;
- breathing problems, shortness of breath;
- suffocation, fainting, signs of hypoxia begin as the disease becomes more severe;
- blanching of the mucous membranes. Over time, they acquire a bluish tint;
- reflex cough during asthma attacks;
- characteristic posture of lack of air: the animal stands with its paws spread and its neck extended;
- pulmonary edema, accumulation of fluid in the pleural cavity;
- paralysis of the hind legs can occur in severe stages, as a symptom of severe thromboembolism;
- sudden death of an animal may be the only symptom of the disease if it occurred in a latent form without obvious symptoms.
Causes of pathology
The primary form of the disease is provoked by genetic mutations that occur during fetal development. Secondary pathology occurs under the influence of a number of predisposing factors, such as:
- poor nutrition;
- endocrine disorders;
- lack of taurine;
- high blood pressure;
- oncological diseases;
- severe intoxication;
- lung damage;
- congenital anomalies of myocardial development;
- general anesthesia;
- chronic infectious foci.
High blood pressure in cats contributes to the development of hypertrophic cardiomyopathy
A predisposing factor in the occurrence of this heart pathology can be the constant presence of helminths in the cat’s body, especially large ones. The fact is that parasites in the process of life produce toxins that can provoke a number of pathologies. If deworming is not carried out on time, the cat will suffer from chronic intoxication, which negatively affects the functioning of the heart.
Diagnosis of the disease
The veterinarian makes a preliminary diagnosis by listening to heart sounds. The basis is systolic murmurs, heart rhythm disturbances (arrhythmias) and gallop rhythm (three-part rhythm, indicating myocardial failure). Clarifying diagnostics includes instrumental examination of the heart muscle:
- Chest X-ray. It will allow you to see pathologically dilated cardiac chambers and pleural effusion in the presence of pulmonary reactions.
- Electrocardiography is needed to detect the presence of arrhythmias and tachycardia. At this stage, a diagnosis of HCM can already be made.
- Ultrasound examination of the heart is the most informative method. It is needed to determine how thick the myocardial wall is. It is possible to determine the amount of blood flow and the presence of blood clots in the arteries.
Cats at high risk of disease should be examined especially carefully. It is determined using special genetic tests that help to see the individual’s individual predisposition.
Treatment of the disease
Complete cure of the disease can only occur as a result of surgical intervention. Conservative treatment is aimed at strengthening the heart muscle and reducing the load on it as much as possible. This will extend the life of the animal and improve its quality of life. Drug therapy is of great importance, although it is equally important to exercise some caution in keeping and caring for a sick animal.
Drug therapy
Cats with this disease that receive adequate treatment usually live quite a long time.
Typically, the treatment regimen is based on drugs that reduce the load on the heart:
- Beta blockers (Atenolol, Propranolol) reduce the number of contractions per unit time, thereby reducing the organ's need for oxygen. Prevent arrhythmia.
- Calcium channel blockers (Diltiazem), by reducing the heart rate, help the hypertrophied myocardial wall to relax and partially recover.
- ACE inhibitors (Enalapril) are used to treat heart failure and reduce high blood pressure.
- Diuretics (Furosemide) are used to prevent congestion in the body, since there is a high probability of pulmonary edema or pleural effusions.
If diuretics are not effective enough, excess fluid is removed from the body surgically by puncturing the chest in the area where there is an accumulation of pleural effusion.
Content Features
Particular attention when caring for a sick animal should be paid to the following points: preventing stressful situations, limiting physical activity and a therapeutic diet.
Therapeutic diet
To prevent stagnation in the body, the animal is offered a salt-free diet. It helps keep blood pressure normal.
The diet must contain medicinal food with the amino acid taurine, which can also be given orally. This will significantly reduce the load on the animal’s heart and prevent the development of heart failure and other unpleasant symptoms.
Treatment of cats with DCM
Hypothermia is common in cats with decompensated DCM.
Therefore, if necessary, it is necessary to provide external heat sources to warm the animal. Pleural fluid is removed by thoracentesis. Once pulmonary edema is controlled, diuretics such as furosemide (1 mg/kg - 0.35 ml) are given orally every 12 hours. Diuretics are given to promote diuresis, as previously described for VSMC. The venoditator nitroglycerin may be useful in cats with severe pulmonary edema. Vasodilators (eg, Benazepril tablet 5 mg orally every 24 hours or pimobendan tablet 1.25 mg orally every 12 hours) may help maximize cardiac output, although with the risk of hypotension. Blood pressure, hydration, renal function, electrolyte balance, and peripheral perfusion should be monitored continuously.
Intravenous digoxin was also used initially in cats with decompensated DCM. Amrinone is a positive inotropic agent with peripheral vasodilating properties, although the exact dosage for cats has not been established. Oral digoxin is the positive inotropic drug of choice for maintenance therapy. Digoxin tablets are commonly used because many cats do not like the taste of digoxin syrup. Toxicity can easily occur, especially if other drugs are used concomitantly, so periodic assessment of serum digoxin levels is required.
Furosemide and vasodilators may reduce cardiac congestion and accelerate the development of cardiogenic shock in cats with DCM. Isotonic saline with 5% dextrose in a 1:1 ratio or other low-sodium solutions can be given intravenously at doses of 20-35 ml/kg/day several times a day or by continuous infusion. Potassium supplementation may be required. Fluid can be given subcutaneously if necessary, although absorption of fluids from the extravascular space may be impaired.
Long-term treatment for cats that survive acute heart failure includes the oral medications pimobendan, furosemide, an ACE inhibitor, digoxin, aspirin (or warfarin), and a taurine supplement or high-taurine diet. Taurine supplementation, 250–500 mg orally every 12 hours, should be administered as soon as possible in cats with low or normal plasma taurine concentrations. Taurine is available in 500 mg capsules from sports and health food stores. Since clinical improvement usually does not begin after 1 to 2 weeks of taurine supplementation, maintaining cardiac treatment is vital. Treatment with aspirin or warfarin is usually used to reduce the risk of thromboembolism. Echocardiographic evidence of improved systolic function is observed in most cats within 6 weeks of starting taurine. Drug therapy may become unnecessary in some cats after 6 to 12 weeks, although it is recommended that resolution of pleural effusion and pulmonary edema be confirmed radiographically before stopping the cat's medication. When echocardiographic measures of systolic function are at or near normal, the amount of taurine may be reduced and therapy eventually discontinued as long as the cat eats a diet known to maintain adequate plasma taurine concentrations (eg, most good commercial diets). ). Dry diets with 1000 to 1200 mg taurine per kilogram dry weight and canned diets with 2000 to 2500 mg taurine per kilogram dry weight are thought to maintain normal plasma taurine concentrations in adult cats. It is also recommended that re-evaluation of plasma taurine concentrations be recommended 2-4 weeks after discontinuation of the drug. DCM due to taurine deficiency in cats that survive one month after initial diagnosis can often be weaned off all or most medications except taurine. These cats are known to have a 50% chance of surviving 1 year. Prognosis for cats with DCM
, not related to taurine or those that do not respond to taurine therapy are very poor.
Thromboembolism in a cat with DCM is a serious and completely unfavorable sign. ^Top
Preventive measures
It is difficult to prevent the primary form of the disease. It is recommended that individuals at risk of such hereditary pathologies not be allowed to breed for breeding in order to exclude the possibility of the appearance of offspring with this pathology.
Owners of Maine Coon and Ragdoll cats are recommended to conduct a special test for their pets to determine its genetic status. Prevention of the secondary form of GPCM should be carried out throughout the life of the animal. It includes the following main steps:
- regular preventive examinations at a veterinary clinic;
- drawing up a balanced diet with a sufficient content of microelements that strengthen the heart muscle (potassium, magnesium) and the amino acid taurine;
- training the heart muscle by stimulating physical activity;
- preventing stressful situations.
The owner can independently assess the condition of his pet. To do this, he must independently determine how many beats per minute his heart makes. A weakened pulse in the femoral artery (less than one hundred beats per minute) may indicate that the animal has heart failure or thromboembolism (blockage of large arteries).
Feline hypertrophic cardiomyopathy is a fairly common disease. The most effective measures to help delay its onset or even prevent it altogether are constant prevention and early diagnosis. Therefore, you should never neglect these measures. In this situation, it is important not to waste time.
Etiology
The cause of primary or idiopathic hypertrophic cardiomyopathy (HCM) in cats is unknown, but inherited pathology likely exists in many cases. The disease appears to be widespread in several breeds, such as Maine Coon, Persian, Ragdoll, and American Shorthair. There are also reports of HCM in littermates and other close relatives of domestic shorthaired cats. An autosomal dominant pattern of inheritance has been found in some breeds. It is known that there are many different gene mutations in people with familial HCM. Although some common human gene mutations do not yet appear to be found in cats with HCM, others may be found in the future. Some researchers (Meurs 2005) have also found a mutation in the myosin binding protein C of myocytes in this breed. Another mutation has been identified in ragdolls; testing for these mutations is currently available (www.vetmed.wsu.edu/deptsVCGL/felineTests.aspx).
In addition to mutations in genes that encode proteins responsible for myocardial contractility and regulatory proteins, possible causes of the disease include increased sensitivity of the myocardium to excess production of catecholamines; pathological hypertrophic response to myocardial ischemia, fibrosis or trophic factors; primary collagen pathology; disorders of myocardial calcium-related processes. Myocardial hypertrophy with focal mineralization occurs in cats with hypertrophic feline muscular dystrophy, which is an X-linked recessive dystrophic disorder similar to Duchenne muscular dystrophy in humans; however, congestive heart failure is uncommon in these cats. Some cats with HCM have high serum concentrations of growth hormone. It is unclear whether viral myocarditis plays a role in the pathogenesis of feline cardiomyopathy. In one study, myocardial samples from cats with HCM were assessed by polymerase chain reaction (PCR) and showed the presence of panleukopenia virus DNA in approximately one third of cats with myocarditis and did not show its presence in healthy control cats (Meurs, 2000).
Pathophysiology
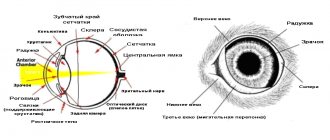
Thickening of the left ventricular wall and/or interventricular septum is common, but the extent and distribution of hypertrophy is variable in cats with HCM. Many cats have symmetric hypertrophy, but some have asymmetric thickening of the interventricular septum and few have hypertrophy limited to the left ventricular free wall or papillary muscles. The left ventricular lumen usually appears small. Focal or diffuse areas of fibrosis occur in the endocardium, conduction system, or myocardium; narrowing of the small coronary arteries may also be present. Areas of myocardial infarction and misalignment of myocardial fibers may be present.
Myocardial hypertrophy and accompanying changes increase the rigidity of the ventricular wall. In addition, early active myocardial relaxation may be slow and incomplete, especially in the presence of myocardial ischemia. This further reduces ventricular compliance and promotes diastolic dysfunction. Ventricular stiffness impairs filling of the left ventricle and increases diastolic pressure. Left ventricular volume remains normal or decreases. Decreased ventricular volume causes decreased stroke volume, which may promote neurohormonal activation. Higher heart rate further affects left ventricular filling by promoting myocardial ischemia, pulmonary venous congestion and edema, shortening the duration of diastolic filling. Contractility or systolic function is usually normal in affected cats. However, some cats gradually develop systolic ventricular failure and ventricular dilatation.
The progressive increase in left ventricular filling pressure leads to increased pressure in the left atrium and pulmonary veins. The result may be progressive enlargement of the left atrium and pulmonary congestion and edema. The degree of left atrium enlargement varies from mild to severe. Thrombi are sometimes found in the lumen of the left ventricle or attached to the wall of the ventricle, although they are more often localized in the left atrium. Arterial thromboembolism is a major complication of HCM, as well as other forms of cardiomyopathies in cats. Some affected cats develop mitral regurgitation. Changes in left ventricular geometry, papillary muscle structure, or systolic motion of the mitral valve (systolic anterior leaflet motion (SAM)) may prevent normal valve closure. Valvular regurgitation increases left atrial size and pressure.
Systolic dynamic obstruction of the left ventricular outflow tract occurs in some cats. This phenomenon is also called hypertrophic obstructive cardiomyopathy or functional subaortic stenosis. Excessive asymmetric hypertrophy of the base of the interventricular septum may be evident on echocardiogram and at autopsy. Systolic outflow tract obstruction increases left ventricular pressure, adversely affects the ventricular wall, increases myocardial oxygen demand, and promotes myocardial ischemia.
Mitral regurgitation increases the tendency for the anterior mitral valve leaflet to move toward the interventricular septum during ventricular systole (SAM). Increased turbulence in the left ventricular outflow tract often produces a systolic murmur of varying intensity in these cats.
Various factors likely contribute to the development of myocardial ischemia in cats with HCM. These include narrowing of the intramural coronary arteries, increased left ventricular filling pressure, decreased coronary artery perfusion pressure, and insufficient myocardial capillary density depending on the degree of hypertrophy. Tachycardia promotes ischemia by increasing myocardial oxygen demand while decreasing diastolic coronary perfusion time. Ischemia impairs early active relaxation of the ventricles, which later increases ventricular filling pressure and, over time, leads to myocardial fibrosis. Ischemia can provoke arrhythmia and possibly chest pain.
Atrial fibrillation and other tachyarrhythmias further impair diastolic filling and increase venous stasis; Particularly harmful are the loss of normal atrial contractions and increased heart rate associated with atrial fibrillation. Ventricular tachycardia or other arrhythmias may lead to syncope or sudden death. Pulmonary venous congestion and edema are caused by increased left atrial pressure. Increased pulmonary venous and capillary pressure causes pulmonary vasoconstriction; Increased pulmonary arterial pressure and symptoms of secondary right-sided congestive heart failure may occur. Over time, some cats with HCM develop refractory biventricular failure with massive pleural effusion. The effusion is usually a modified transudate, although it may be (or become) chylous.
Clinical manifestations
HCM is most common in middle-aged male cats, but clinical signs can occur at any age. Cats with mild disease may be asymptomatic for several years. Symptomatic cats most often exhibit varying degrees of respiratory symptoms or symptoms of acute thromboembolism. Respiratory symptoms include tachypnea; activity-associated shortness of breath; dyspnea and very rarely cough (which can be confused with vomiting). The onset of the disease may be acute in sedentary cats, even if pathological changes develop gradually. Sometimes lethargy and anorexia are the only manifestation of the disease. Some cats experience syncope or sudden death in the absence of other symptoms. Stressors such as anesthesia, surgery, fluid administration, systemic illness (eg, hyperthermia or anemia), or transportation may precipitate heart failure in compensated cats. Asymptomatic disease is detected in some cats by detecting a heart murmur or galloping rhythm on routine auscultation.
Systolic murmurs caused by mitral regurgitation or left ventricular outflow tract obstruction are often detected. Some cats have no audible murmur, even those with severe ventricular hypertrophy. A diastolic gallop sound (usually S4) may be audible, especially if heart failure is evident or threatened. Cardiac arrhythmias are relatively common. The femoral pulse is usually strong. with the exception of cases of distal aortic thromboembolism. The heartbeat is often increased. Increased respiratory sounds, pulmonary rales and sometimes cyanosis accompany severe pulmonary edema. Crackles in the lungs are not always heard with pulmonary edema in cats. Pleural effusion usually attenuates ventral lung sounds. Physical examination may be normal in subclinical cases.
Diagnosis
Radiography
Radiographic features of HCM include enlargement of the left atrium and varying degrees of enlargement of the left ventricle. The classic valentine-shaped heart appearance in dorsoventral and ventrodorsal views is not always present, although the position of the left ventricular apex is usually preserved. The cardiac silhouette appears normal in most cats with mild HCM. Dilated and tortuous pulmonary veins may be seen in cats with chronic increases in pulmonary vein and left atrium pressure. Left-sided congestive heart failure causes patchy infiltrates expressed to varying degrees with interstitial or alveolar pulmonary edema. Radiologically, the distribution of pulmonary edema is variable; A diffuse or localized distribution within lung fields is usually found, as opposed to the characteristic hilar distribution of cardiogenic pulmonary edema in dogs. Pleural effusion is common in cats with advanced or biventricular congestive heart failure.
Electrocardiography
The majority of cats with HCM (up to 70%) have electrocardiographic abnormalities. These include abnormalities characterized by left atrial and left ventricular enlargement, ventricular and/or (less commonly) supraventricular tachyarrhythmias, and signs of left bundle branch block. Atrioventricular conduction delay, complete atrioventricular block, or sinus bradycardia sometimes occur.
Echocardiography
Echocardiography is the best method for diagnosing and differentiating HCM from other diseases. The extent of hypertrophy and its distribution within the free wall of the left ventricle, interventricular septum and papillary muscles is revealed in M-mode and B-mode echo studies. Doppler ultrasound may demonstrate left ventricular systolic and diastolic abnormalities.
Widespread myocardial thickening is common, and hypertrophy is often asymmetrically detected in the left ventricular free wall, interventricular septum, and papillary muscles. Focal zones of hypertrophy also occur. Using B-mode helps ensure that the scanning direction is correct. Standard M-Mode measurements should be taken, but areas of thickening outside these standard positions should also be measured. Diagnosis at an early stage of the disease may be presumptive in cats with mild or only focal thickening. False-positive thickening (pseudohypertrophy) can occur with dehydration and sometimes with tachycardia. False diastole thickness measurements also occur when the ultrasound beam does not cross the wall/septum perpendicularly and when measurements are not taken at the end of diastole, which can occur without a simultaneous ECG, or when the use of B-mode is insufficient for a good measurement. A thickness of the free wall of the left ventricle or interventricular septum (correctly measured) of more than 5.5 mm is considered pathological. Cats with advanced HCM have a diastolic left ventricular septal or left ventricular free wall thickness of 8 mm or more, although the degree of hypertrophy does not necessarily correlate with the severity of clinical signs. Doppler assessments of diastolic function, such as isovolumic relaxation time, mitral inflow, and pulmonary venous velocity, as well as Doppler tissue imaging techniques, are increasingly being used to characterize disease.
Hypertrophy of the papillary muscles may be severe and obliteration of the left ventricle during systole has been observed in some cats. Increased echogenicity (brightness) of the papillary muscles and subendocardial areas is usually a marker of chronic myocardial ischemia with resultant fibrosis. Left ventricular shortening fraction is usually normal or increased. However, some cats have mild to moderate left ventricular dilatation and reduced contractility (contraction fraction 23-29%; normal contractility fraction 35-65%). Sometimes there is enlargement of the right ventricle and pleural or pericardial effusion.
Cats with dynamic left ventricular outflow tract obstruction also often have mitral valve SAM or early closure of the aortic valve leaflets on M-mode imaging. Doppler ultrasonography can demonstrate mitral regurgitation and turbulence in the left ventricular outflow tract, although positioning the ultrasound beam along the blood stream with maximum ventricular ejection velocity is often difficult and it is easy to underestimate the systolic gradient.
Enlargement of the left atrium can range from mild to severe. Spontaneous enhancement (rotation, smoke echo) is seen within the enlarged left atrium in some cats. It is assumed that this is the result of blood stasis with cell aggregation and is a precursor to thromboembolism. Thrombosis is sometimes visualized within the left atrium, usually in its appendage.
Other causes of myocardial hypertrophy must be excluded before a diagnosis of idiopathic HCM is made. Myocardial thickening may also occur due to infiltrative disease. Variations in myocardial echogenicity or wall irregularity may be detected in such cases.
Excess connective tissue appears bright. linear echoes within the left ventricular cavity.
Clinicopathological features
Cats with moderate to severe HCM have high circulating concentrations of natriuretic peptides and cardiac troponins. In cats with congestive heart failure, plasma concentrations of tumor necrosis factor (TNF) have been found to be increased to varying degrees.
Picture 1
Radiographic findings in feline HCM. Lateral (A) and dorsoventral (B) views demonstrating left atrial enlargement and mild ventricular enlargement in a male domestic shorthair cat. Lateral © view in a cat with HCM and severe pulmonary edema